
January 13, 2022
Dear Colleague:
This communication outlines the new therapeutics that are available for the immunocompromised and patients with Covid-19 who are at risk for progression to severe Covid-19. For a Clinical Decision Tree App and Outpatient Treatment Prioritization Table,
click here.
Due to limited supply of resources, the Monoclonal Antibody Collaborative (MAC) urges providers to refer patients who fall into Tier I and Tier 2 categories of the
NIH Outpatient Treatment Prioritization Table. The MAC is following NIH guidance.
statement-on-anti-sars-cov-2--12-23-2021.pdf (nih.gov)
Monoclonal Antibodies (MAB):
For Treatment:
Omicron has numerous mutations in the spike protein that has reduced the efficacy of several of the monoclonal antibodies, especially bamlanivimab plus etesevimab and casirivimad plus imdevimab. Sotrovimab does have 85% hospitalization risk reduction against omicron. For referral to the Regional Infusion Center (RIC) for MAB treatment,
click here.
For Preexposure Prophylaxis:
The MAC clinic is treating Central Texas patients eligible for Evusheld, a monoclonal antibody for preexposure prophylaxis used in adults and children 12y or older weighing at least 40kg, with moderate to severe immunocompromise or who receive immunosuppressive medications and may not mount an adequate response to a Covid-19 vaccination. Evusheld is not for treatment of Covid-19.
Providers are required to have a clinical conversation regarding risk and benefits with their patient before completing and submitting the referral form. For HIPAA compliant referral to the Prep MAC Clinic for Evusheld therapy,
click here.
Once the entire form is complete and submitted, the provider should call 512-972-5545 to schedule an appointment with Austin Public Health staff. The hotline will be active Monday-Friday 9am-5pm. This hotline is only for providers who have submitted a completed form, if a patient calls, they will be referred to their provider.
EVUSHELDTM – Clinical Considerations
• Not authorized for treatment of COVID-19, nor for post-exposure prophylaxis of COVID19 in individuals who have been exposed to someone infected with SARS-CoV-2.
• Pre-exposure prophylaxis with EVUSHELD is not a substitute for vaccination in individuals for whom COVID-19 vaccination is recommended.
• In individuals who have received a COVID-19 vaccine, EVUSHELD should be administered at least two weeks after vaccination.
• Examples of medical conditions or treatments that may result in moderate to severe immune compromise and an inadequate immune response to COVID-19 vaccination are provided in the Health Care Provider Fact Sheet.
Health Care Provider Fact Sheet:
https://www.fda.gov/media/154701/download.
Overview of Outpatient Therapeutics:
Below is an algorithm that can be used as a decision tree for use of the outpatient therapeutics that are available in limited supply for treatment of Sars-Cov-2.
PowerPoint Presentation (idsociety.org)
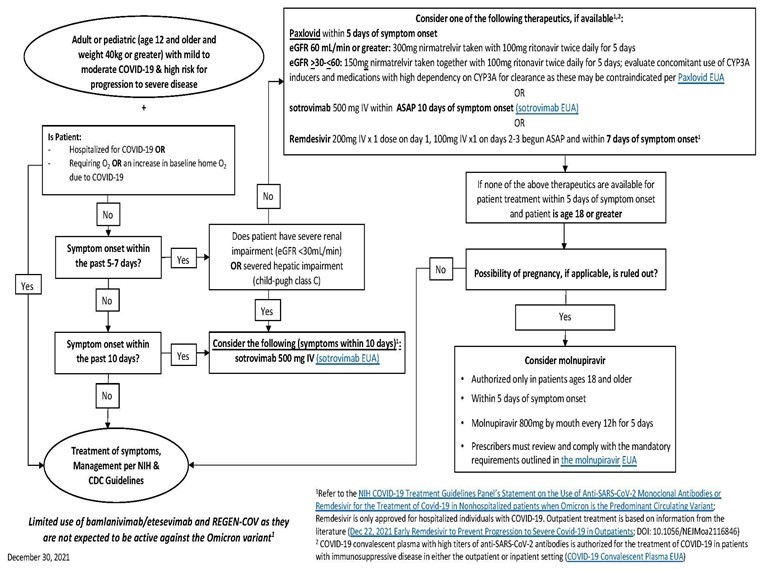
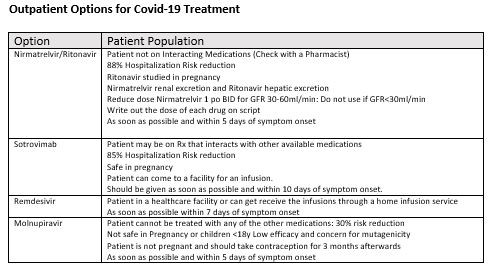
The slides in this letter are
from a recent Covid-19 update webinar that is available on line at the
following link CDC/IDSA Clinician Call: COVID-19Treatment Updates Plus the Latest on Omicron (idsociety.org).
These new medications are in
short supply now, but production has been ramped up and supply is to increase
in the coming months. Thank you for your commitment to patients in Central
Texas.
Stay Safe,
Desmar Walkes, MD
Medical Director/Health Authority
City of Austin / Travis County
Office 512-972-5097
Cell 737-262-9663
Email Desmar.walkes@austintexas.gov